Legal Manufacturer Definition Medical Devices

The pivotal provision that qualifies a medical device manufacturer as such is the characteristic of placing the medical device on the market under his own name the manufacturer clearly is responsible for the medical device manufacturing operations though the functions described can be variously delegated or subcontracted.
Legal manufacturer definition medical devices. If you are manufacturing a medical device you must follow the specific directive for your type of. A manner that meets this definition it will be regulated as. Manufacturer makes by chemical physical biological or other procedures any article that meets the definition of device in section 201 h of the federal food drug and cosmetic fd c act. This economic operator the own brand labeller meets the definition of manufacturer as set out in the medical devices directives.
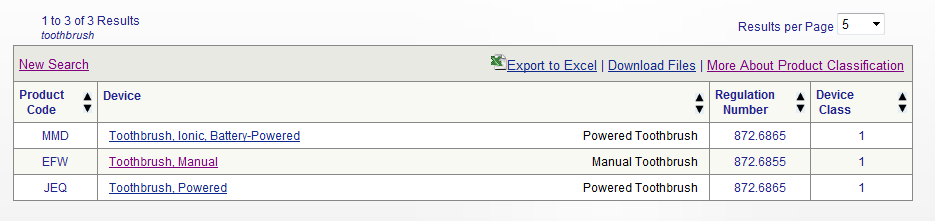
1 medical device means any instrument apparatus appliance software implant reagent material or other article intended by the manufacturer to be used alone or in combination for human beings for one or more of the following specific medical purposes. How to comply with the legal requirements. Definition of a medical device. Own brand labeller or private labeller are therefore considered as the legal manufacturer.
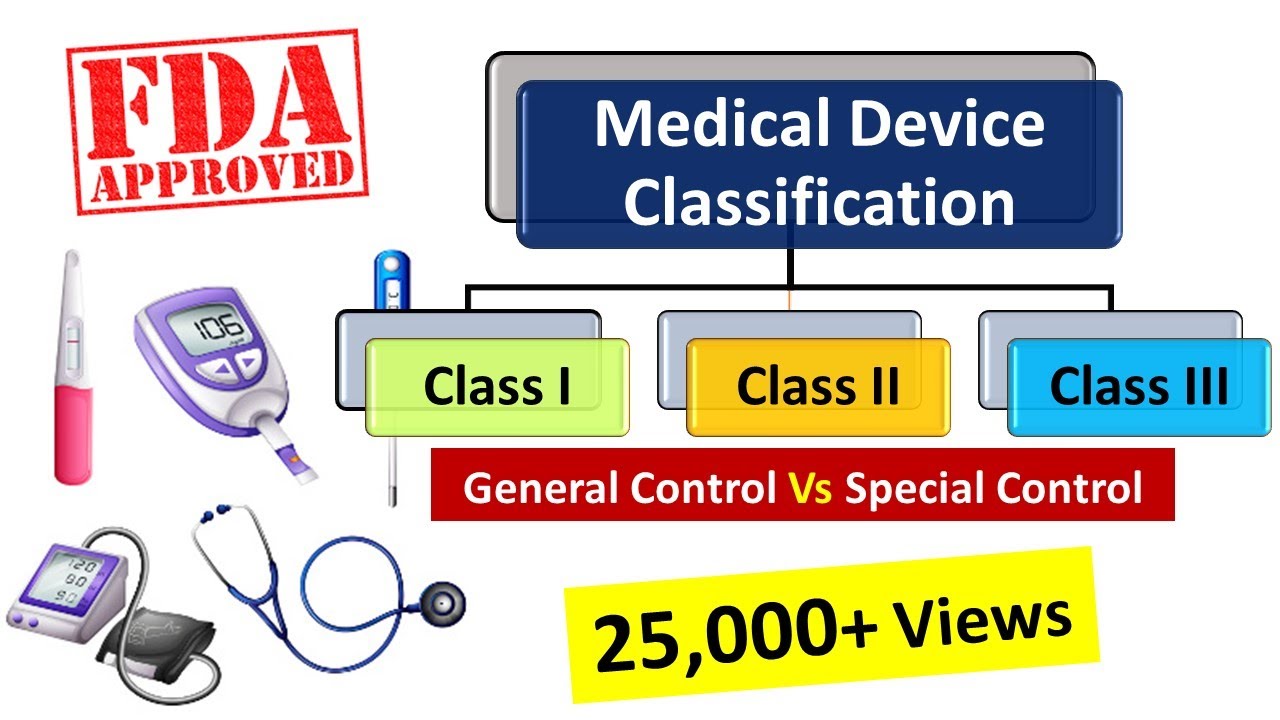
With the publication of europe s new medical device regulation eu 2017 745 and in vitro diagnostic regulation eu 2017 746 some companies that contract manufacture or relabel devices are wondering if they will be considered the legal manufacturer of the device they sell much of the confusion around this issue stems from the assumption that there can be only one legal manufacturer. In accordance with the medical devices regulation eu 2017 745 a manufacturer is defined as. Medical device classification pre market submission medical device registration and listing 510 k pma medical device labeling.